by Gaetana Tonti, bioscience researcher and META-Health Master Practitioner
Metastasis derives from the Greek META: beyond, and STASIS: to place, and is used to describe the spread of cancer cells from the primary site to another organ/part of the body. To do this, cancer cells from the first site need to move and invade the secondary site. There has been some debate about the existence of cancerous cells circulating in peripheral blood, especially in the META-medicine world; so it might be interesting to briefly report the most recent discoveries and hypotheses, and discuss META-stases from a META-perspective.
Even though the development of disseminated metastatic diseases has been traditionally viewed as a sequential rather than concurrent process, (i.e. the disease initially occurs at the primary site, followed by local growth with dissemination to distant sites much later in time (1)), emerging data is challenging this theory (2, 3), suggesting that the presence of CTCs and DTCs is a very early, if not concomitant phenomenon, to the onset of the primary tumor.
Bone marrow has revealed to be a common homing organ for metastatic cells, independent of the primary tumor site and the pattern of overt metastases (4). This suggests a causal link and a specific meaning for the presence of DTCs in the bone marrow. In fact, DTCs in bone marrow have been detected in all solid tumor types suggesting that the bone marrow might be a preferred reservoir for blood-borne DTCs.
However, up to now it is unknown which environmental and internal factors can promote the recirculation of DTCs from the bone marrow niche into other distant organs – such as liver or lungs, which are common sites of metastatic spread. These tumor cells detached from the primary tumor flow in the peripheral blood and reach the bone marrow in a dormant state until internal and/or external signals enable them to move and grow out to overt metastases at different organs (5-7).
Little is known about these dormant cells and the conditions required—environmental factors (e.g., immune system, dietary changes…..stress, emotional traumas….. repair of damaged tissues in a regeneration phase)—for their ‘‘awakening’’ from the dormant phase into the dynamic phase of metastasis formation. According to conventional science the dormant state might be disturbed by both changes within the DTCs (e.g., additional DNA mutations) and the surrounding microenvironment (e.g., decrease in immune surveillance or increased angiogenetic potential (8, 9).
But what if a more bio-logical and meaningful reason existed for the explanation of this phenomenon?
Messages from the environment
Cancer cell behaviors are not regulated by a linear series of commands, but rather by networks of molecular interactions that involve positive and negative reinforcement. Switching between different cell fates that are critical for cancer (e.g., growth, differentiation, increased motility) can be triggered in normal and transformed cells, by changes in extracellular matrix (ECM) structure and cell shape distortion (10-14), ‘non-specific’ chemical solvents and electrical ion flows (15-17) that influence multiple gene activities, as well as by distinct molecular factors or specific gene mutations.
There are many studies in experimental systems suggesting that cancers can be induced to become quiescent, differentiate, die or form completely normal tissues, if provided with the correct set of complex signals, as conveyed by embryonic tissues or other microenvironmental cues (18). This means that for some reason the environment surrounding the primary tumor becomes ‘‘activated,’’ and it can release cancer and metastasis-inducing signals.
This scenario represents a clear example of the acquisition of highly malignant cell biological traits through an adaptive mechanism, arguing strongly for adaptation/response as the prime driving force that underlies the acquisition of highly malignant traits by neoplastic cells.
A new META-perspective on cancer and META-stases
On these bases a very challenging hypothesis has been proposed:
- Cancer as a natural wound healing related process where the outcome of the cancer mechanism is either healing the wound or exhausting the whole system (death).
The wound signaling molecules present in a tissue cause over-expression of the existing cancer-related genes, leading to the changes of certain chromosomes and cancer cell traits (19). Therefore, a (wound–oncogene–wound healing (WOWH)) can be logically speculated: cancer formation is a natural process that organisms have used in wound healing. Briefly, when defined wounds occur in mammals, the body starts the complicated wound healing process.
Molecules such as growth factors, cytokines, and other proteins from the cells in the wound area interrupt the balance of normal molecular metabolism, leading to the activation of corresponding cancer-related genes and inducing cancerization in some cells. The cells with activated genes can secrete molecules to recruit other cells, stimulate cell proliferation, and enhance cell differentiation to repair the wound. Mostly, the wound is healed after above efforts.
However, if the wound conditions are still persistent this WOWH mechanism will not stop and more cancer cells (over-activation of the canre-related genes transformed normal cells into malignant cells (21, 21) are divided to secrete more molecules for wound healing, forming a clinical cancer mass. After the wound is healed, the molecules of a healed environment initiate cancer cell differentiation or death.
This model leads also to a new concept of metastasis
Based on the idea that cancer cells are present to repair a wound, a META explanation of metastasis is that cancer cells in the primary tumor site can “sense” the presence of wounds at distant sites and these cancer cells can migrate through the circulation in order to heal the secondary wound. At this point it might be interesting to define the term “wound”: is it only a physical wound?! But from a META-perspective we know that there is never a physical wound without an emotional/spiritual wound.
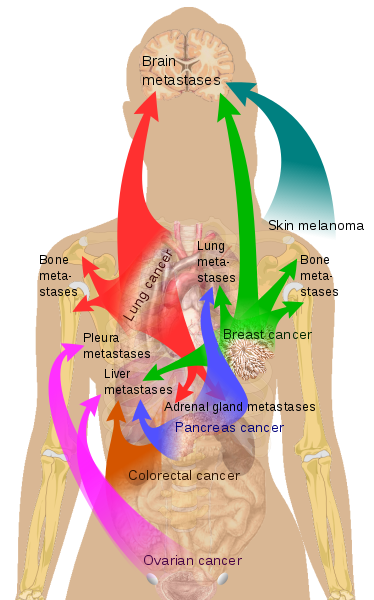
Similarly some people may experience a survival-existence conflict, worrying if they will survive. In this case the liver will be involved. Again, in the stress phase it will have an increase in cell activity/number, leading to a proliferation of the adeno-cells of the liver. When diagnosed with primary cancer, many people can also feel worthless, incapable, not able to sustain the situation, and in this case the bone tissue will react. As discussed previously, metastatic cells invade the bone marrow and reside in a dormant state. When triggered they cause bone tumor and following the resolution there will be calcification of cells and the bone will be stronger than before (for bones the bio-logical meaning is in the cell increase and strengthening of the bone at the end of the regeneration phase).
 The final question could be “Why the cancer cells from the primary tumor migrate to the bone marrow and remain in a dormant state, waiting to be awakened and eventually migrate to secondary sites?” According to the WOWH theory these primary cancer cells might sense an emotional wound/stress, and from a META-perspective it would be a deep wound, that hits the core of who we are, the sense that lives gives to us or that we give to life… the inmost, the essential part of who we are… the marrow. I view this event as if when a person develops a primary cancer, our bodily intelligence wants to protect us, sending these primary cells directly to our core, our marrow; these cells remain there, dormant, ready to move to other organs when needed. Our body is a highly intelligent organism where every event/symptoms has a meaningful reason to exist.
The final question could be “Why the cancer cells from the primary tumor migrate to the bone marrow and remain in a dormant state, waiting to be awakened and eventually migrate to secondary sites?” According to the WOWH theory these primary cancer cells might sense an emotional wound/stress, and from a META-perspective it would be a deep wound, that hits the core of who we are, the sense that lives gives to us or that we give to life… the inmost, the essential part of who we are… the marrow. I view this event as if when a person develops a primary cancer, our bodily intelligence wants to protect us, sending these primary cells directly to our core, our marrow; these cells remain there, dormant, ready to move to other organs when needed. Our body is a highly intelligent organism where every event/symptoms has a meaningful reason to exist.
REFERENCES
- Guislaine Barri`ere, Michel Tartary, and Michel Rigaud . Epithelial Mesenchymal Transition: A New Insight into the Detection of Circulating Tumor Cells. Int Schol Res Net ISRN Oncol 2012, doi:10.5402/2012/382010
- Mego M, Mani SA, Cristofanilli M: Molecular mechanisms of metastasis -clinical applications. 2010, 7(12):693-701.
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH-F, Norton L, Massague J: Tumor self-seeding by circulating cancer cells. Cell 2009, 139:1315-1326.
- Pantel K, Cote RJ, Fodstad O: Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst 1999, 91: 1113–1124.
- Allan AL, Vantyghem SA, Tuck AB, Chambers AF: Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis 2006, 26: 87–98.
- Alix-Panabieres C, Muller V, Pantel K: Current status in human breast cancer micrometastasis.Curr Opin Oncol 2007, 19: 558–563.
- Pantel K, Schlimok G, Kutter D, Schaller G, Genz T, Wiebecke B, Backmann R, Funke I, Riethmuller G: Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Res 1991, 51: 4712–4715.
- Naumov GN, Bender E, Zurakowski D, et al. A model of human tumor dormancy: an angiogenic switch from the non angiogenic phenotype. J Natl Cancer Inst 2006, 98:316.
- Marches R, Scheuermann R, Uhr J. Cancer dormancy: from mice to man. Cell Cycle 2006, 5: 1772
- Kalluri R, Weinberg RA, “The basics of epithelial-mesenchymal transition,” Journal of Clinical Investigation, 2009, 119,6: 1420–1428,
- Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher’s conceptual friend and foe. American Journal of Pathology, 2009, 174, no. 5: 1588–1593.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell, 2000, 100:57–70.
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer, 2002, 2: 563–572.
- Christina Scheel, Tamer Onder, Antoine Karnoub, et al. ADAPTATION VERSUS SEPECTION: THE ORIGINS OF METASTATIC BEHAVIOUR; CANCER RES, 2007, 67:11476-11480.
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126:677–689.
- Huang S, Eichler G, Bar-Yam Y, Ingber DE. Cell fates as high-dimensional attractor states of a complex gene regulatory network. Phys Rev Lett 2005, 94:128701.
- Hellmann P, Grummer R, Schirrmacher K, Rook M, Traub O, Winterhager E. Transfection with different connexin genes alters growth and differentiation of human choriocarcinoma cells. Exp CellRes 1999, 246:480–490.
- Maffini MV, Soto AM, Calabro JM, Ucci AA, Sonnenschein C. The stroma as a crucial target in rat mammary gland carcinogenesis. J Cell Sci 2004, 117:1495–1502.
- Zhou, Y., Ma, B. G., & Zhang, H. Y. Human oncogene tissue-specific expression level significantly correlates with sequence compositional features. FEBS Letters, 2007, 581: 4361–4365.
- Haber, M., & Stewart, B. W. Oncogenes. A possible role for cancer genes in human malignant disease. The Medical Journal of Australia, 1985, 142: 402–406.
- Mitsushita, J., David Lambeth, J., & Kamata, T. The superoxide-generating oxidase Nox1 Is functionally required for Ras oncogene transformation. Cancer Research, 2004, 64:3580–3585
You can read the full scientific paper here!>
pictures:
wikipedia, user Mikael Häggström
wikipedia, userJames Heilmann, MD
Pixabay, user Pexels
